Link to final published paper
Download this White Paper (revised version)
Download this White Paper (original version)
Download Slides
Continuous Bioprocessing

Konstantin B. Konstantinov, Charles L. Cooney
ABSTRACT
There is a growing interest in realizing the benefits of continuous processing in biologics manufacturing, which is reflected by the significant number of industrial and academic researchers who are actively involved in the development of continuous bioprocessing systems. These efforts are further encouraged by guidance expressed in recent FDA conference presentations. The advantages of continuous manufacturing include sustained operation with consistent product quality, reduced equipment size, high volumetric productivity, streamlined process flow, low process cycle times and reduced capital and operating cost. This technology, however, poses challenges, which need to be addressed before routine implementation is considered. This paper, which is based on the available literature and input from a large number of reviewers, is intended to provide a consensus of the opportunities, technical needs and strategic directions for continuous bioprocessing. The discussion is supported by several examples illustrating various architectures of continuous bioprocessing systems.
Keywords: Continuous bioprocessing, perfusion cell culture, continuous purification, integrated continuous biomanufacturing
1. Introduction
Process intensification through conversion from batch to continuous manufacturing has been applied effectively in industries such as steel casting[1], petrochemical, chemical, food, and pharmaceuticals with great effectiveness.[2],[3],[4],[5],[6] Despite vast differences between the product types, the advantages of continuous over batch manufacturing are consistent and include steady state operation, reduced equipment size, high volumetric productivity, streamlined process flow, low cycle times, and reduced capital cost.[7] An example of industrial importance in the therapeutic field is the ongoing project at the Novartis - MIT Center for Continuous Manufacturing that targets a holistic redesign of the pharmaceutical manufacturing process to achieve fully integrated end-to-end continuous flow.[8]
There is a growing interest in realizing the benefits of continuous processing in biologics manufacturing. To this end, a significant number of industrial and academic researchers are actively involved in the development of continuous processing systems.[9],[10] The results reported so far point to the transformative potential of this technology on the manufacturing of biological drugs. The development efforts are further encouraged by guidance expressed in recent FDA conference presentations.[11],[12]
Continuous bioprocessing technology, as a paradigm shift in biologics manufacture, will benefit from standardizing on common terminology, as well as from the alignment of expectations and goals. An objective of this document is to propose such a common understanding and to define strategic directions for further development that will address the current challenges and technological gaps. While the concept of fully continuous bioprocessing will likely face some skepticism, it is important to consider the lengthy but highly successful evolutionary path of the continuous manufacturing in other industries1,7, which is seen today as a “disruptive technology” that moved these businesses to a new level.[13],[14]
2. Definitions
As with other industries, the classification of a biomanufacturing system as continuous depends on the nature of the unit operations and its integration into the final system. Therefore, it is appropriate to first provide a working definition of the term “continuous unit operation”.
A unit operation is continuous if it is capable of processing a continuous flow input for prolonged periods of time. A continuous unit operation has minimal internal hold volume. The output can be continuous or discretized in small packets produced in a cyclic manner.
A process is continuous if it is composed of integrated (physically connected) continuous unit operations with zero or minimal hold volume in between. To emphasize that all the unit operations are continuous and integrated, such processes are also referred to as fully continuous or end-to-end continuous. A process is hybrid if it is composed of both batch and continuous unit operations.
Continuous processing is a rich technical field that encompasses various concepts, such as flow, systems approach, integration and model-based control. For further discussion of these and other related topics see Badman & Trout.[15]
3. Challenges of current biomanufacturing technology and the opportunities offered by continuous processing
The overarching business drivers for accelerated development times and cost control under stringent quality/regulatory requirements continue to dominate the biotechnology industry. At present, biopharma companies often need to flexibly accommodate large-, mid- and small-volume drugs (e.g., niche or orphan drugs), preferably within the same manufacturing facilities. The same is true for therapeutic proteins of different natures, for example stable proteins, such as antibodies, and highly complex (less stable) proteins, such as recombinant enzymes or blood-factors. Furthermore, the ability to rapidly adjust production capacity to accommodate fluctuating and/or mis-forecasted market demands is also needed. Mounting pressure to reduce cost is impacted by growing competition from biosimilar products and by government regulations. As a result, concerns about the long term sustainability and reproducibility of the traditional batch manufacturing facility model with multiple 10-20kL batch bioreactors and downstream trains involving large chromatographic columns have been repeatedly raised. As a consequence of major improvements in upstream product titers and the identification of high potency products, a trend towards the utilization of smaller, possibly single-use bioreactors and purification columns has emerged, which addresses some, but not all, of the limitations with current technology. Below, we enumerate the main challenges of current batch or hybrid biomanufacturing systems, and review the opportunities offered by integrated continuous processing to address these issues.
3.1. Cost
High capital investment costs are associated with traditional stainless steel fed-batch facilities due to larger equipment size and low equipment utilization rates. Continuous processing offers significant opportunity to reduce capital costs through radical reduction of facility and equipment footprints that result from: small bioreactors and chromatography columns; elimination of intermediate hold tanks and non-value-added unit operations; very high volumetric productivity; high equipment utilization rate; single-use technology. The volume of the continuous bioreactors and purification columns can be multi-fold smaller than the corresponding fed-batch equipment due to significantly higher cell density in perfusion systems and the downstream equipment utilization rate. These small footprint facilities can be constructed and commissioned more expediently than traditional large-scale facilities. Additional savings in operating cost are also expected from the improved resin capacity utilization and accompanying decreases in buffer volumes that are offered by continuous chromatography systems. The functionally closed process will require lower environmental class and therefore drive down capital and QC environmental testing costs as well. Other cost savings will occur through an increase in automation and reduction in operator labor. Further improvements in perfusion processes to increase MAb titer through process and media optimization are needed to achieve COG competitive with current fed-batch processes. As media cost is reduced through systemic development, its impact on COG will become less significant.
3.2. Flexibility
The growing diversity of product pipelines within the industry (including large-volume and small-volume drugs; mAb and non-mAb products) and associated market complexity create a need for production flexibility. Continuous processing offers a significant advantage as it allows rapid capacity adjustments through “numbering up” (addition/removal of parallel production lines) when compared to traditional volumetric scale up. The multi-fold smaller and potentially mobile equipment that is used in continuous processing further enhances this advantage. Another scaling factor in continuous processing is time – the duration of the process can be modulated based on product demand. In combination, these factors allow the utilization of a single bioreactor scale of a few hundred liters for the production of small-volume drugs (<10kg/year) and large-volume drugs (>100kg/year). Furthermore, the reduced equipment footprint and the universality of the platform allows high process mobility and portability, either within the same facility or between different manufacturing sites, which can be strategically distributed to serve local markets.
3.3. Standardization
The diversity of biological products has resulted in the development of a variety of production systems with limited standardization. For example, relatively stable proteins (such as a MAb) are typically produced in fed-batch systems, while less stable molecules (such as blood factors or enzymes) are produced in hybrid systems (perfusion upstream and batch downstream). There is also significant diversity in production systems even within the same technological category, which complicates knowledge management, technology transfer, speed of development, and the ability to capture incremental process improvement. Continuous bioprocessing offers the opportunity to implement standardization, i.e. all biological drugs are manufactured in a common manner. Multi-product facilities then can be designed using a standardized continuous platform. Furthermore, due to small equipment size, full process standardization can now be realized across process development, clinical production, launch and commercial manufacturing, as identical equipment and control systems can be used in all these areas. Standardization will also facilitate compliance with local government regulations in emerging markets, as small, predesigned facilities can be rapidly and inexpensively built in such regions in a decentralized operating model.
Furthermore, standard bioreactors of modest volume can be used for the production of both small-volume and large-volume biologics. For example, a single 500L bioreactor operated continuously at cell density of 80e6 cells/mL for a total of 280 days/year would produce more than 400 kg/year at crude harvest, assuming cell specific productivity of 40pg/cell-day. The same 500L perfusion bioreactor can be used in much shorter campaigns for the production of low-volume drugs. By scaling with longer production cycles a range of market demands can be met.
Additional improvements can be achieved through equipment interchangeability and standardization of interfaces between continuous process equipment. Due to the small, mobile nature of the directly connected continuous unit operations, interface standardization is facilitated by single use technology (e.g., disposable tubing, tubing sealers and tubing welders).
3.4. Speed of scaling
Historically, scale-up and transfer to manufacturing has been a time consuming activity, often associated with the identification of various technical challenges. The utilization of a standard production platform for protein drugs would facilitate and minimize technology transfer activities. This is achieved by the equivalency of scale and equipment for pilot, clinical, and commercial production. Training and validation would also be faster, as the systems will be well understood and characterized, and proper models can be built and common validation protocols can be used. Equipment cleaning requirements will be reduced. The elimination of non-value unit operations and intermediate hold steps will also have a positive impact on time-to-market. It is clear, however, that such advantages can become more evident with the maturation of the platform, both in terms of technology and regulatory approval procedures.
The physical connection and prolonged uptime of all continuous unit operations during scale-up and validation enable the collection of substantial amounts of data offering an unprecedented opportunity to learn about the bioprocess as an integrated system. Over time, the generation of such deep knowledge will enable faster process development and system deployment. One needs to assure consistency of performance across time, along with an understanding of the minimum scale that make continuous bioprocessing attractive. Production over varying periods of time has to be validated as well to provide additional operational flexibility.
3.5. Quality
Due to extended bioreactor residence time, fed-batch technology is only used for the manufacture of stable proteins. If produced in this mode, labile proteins would either degrade or lose some of their quality attributes. Continuous technology allows a significant decrease of the molecule residence time in the bioreactor, which enables the production of any kind of protein with uncompromised quality. It is worth noting that there may be quality benefits even with the production of stable proteins (e.g. mAb’s), as glycosylation profiling changes (less modified and more consistent glycoforms profile) have been reported in batch systems, most likely due to post secretion enzymatic modification or degradation.[16],[17],[18] Modifications, such as deamidation, that are associated with prolonged molecule exposure to bioreactor pH and temperature conditions also can be minimized with continuous cultivation. The elimination of intermediate hold steps further reduces the risk of product degradation and eliminates the need of long-term stability studies. Another advantage is that continuous bioreactors typically operate at high cell culture viability, which in turn results in higher productivity, lower impurity levels in the harvest, and potentially simplified product purification schemes. Since the sustained production is done with close attention to process control, this facilitates the application of QbD principles. The high degree of automation associated with continuous processing further increases operational consistency as it minimizes manual operations and subjective decision-making. The upstream and downstream closed system operation needed for long-term continuous runs would further reduce bioburden risk, which is significant in existing downstream facilities.
4. Technology needs
4.1. Different requirements for upstream and downstream
Continuous perfusion bioreactors have been employed for decades for the commercial manufacture of biologics. In these “hybrid” systems, the bioreactor equipped with a cell retention device is the only continuous unit operation. The downstream part of the process consists of multiple batch unit operations. While significant knowledge with continuous bioreactor operation has been accumulated over the years, the experience with continuous downstream operations is limited. Due to the different levels of maturity, technological needs for upstream and downstream development are rather different.
Technology evolution in a continuous upstream system is expected to be incremental within the existing process architecture. The established bioreactor-cell retention device configuration offers significant advantages, and will likely remain conceptually the same. Improvements within this framework are, however, necessary in order to reach the full potential of the continuous platform. These include the development of robust and stable cell lines that maintain high productivity over prolonged time periods (e.g., 2-3 months), the design of media formulations to support high cell density (e.g., capable of supporting >50e6 cells/mL at perfusion rate of 1-2 reactor vol/day), as well as optimization of bioreactor conditions to provide the high cell density at high viability and positive growth rate. Various enabling technologies need to be developed and optimized, such as automatic cell density control, efficient oxygenation and ventilation, foam control, etc. As bioreactor operation at cell densities above 100e6 cells/mL has already been demonstrated[19], achieving these targets at industrial scale within the continuous upstream technological infrastructure is a realistic near term goal.
The current situation with downstream is quite different, as there is limited experience with continuous protein purification, especially at manufacturing scale. Until recently, equipment for continuous protein purification operations was not available. Today, small- and pilot-scale continuous chromatography systems are offered by several equipment manufacturers (Novasep, Pompey, France; Tarpon, Worcester, MA; Semba, Madison, WI; GE Healthcare, Piscataway, NJ; Knauer, Berlin, Germany; ChromaCon, Zurich, Switzerland). As these systems are new, their adoption by the biotechnology community as routine tools will take time to evaluate, validate and implement. Significant experience needs to be accumulated in process scale up prior to adoption at full scale. Most unit operations employ multicolumn technology utilizing time- or UV-based switching logic that enables the system to automatically process continuous flow. Other, less mature technologies also may be considered, such as annular chromatography[20] or Continuous Countercurrent Tangential Chromatography.[21] In addition to the chromatography steps, novel downstream unit operations are needed, such as continuous viral inactivation/removal and UF/DF, among others. An important design requirement for all continuous downstream unit operations is their ability to operate over prolonged periods under stringent bioburden control conditions.
A major lesson from the development of continuous processes in pharmaceuticals and other industries is that one does not convert a batch process to continuous by simply connecting together existing batch equipment, but rather by designing new fit-for-purpose unit operations and processes. This places a strong dependence on the technology suppliers. There must be broad support by the technology vendors, who have to develop the equipment suitable for commercial continuous processing.
4.2. Standardization of downstream process architecture
Additional challenges emerge with standardization of the downstream process architecture. While the purification of mAbs is relatively well defined, (e.g. protein-A capture), this is not the case with non-mAb molecules. The batch purification trains vary significantly in number and type of chromatography columns, viral inactivation methods, deployment of membrane steps and final formulation. One possible avenue towards the development of a universal downstream architecture is the rational design of highly specific ligands for the capture step analogous to Protein A in mAb purification. While so far this approach has been challenging, the availability of such robust and specific ligands will help standardize the remaining downstream unit operations, ideally utilizing no more than three chromatographic steps.
As today’s continuous downstream unit operations are in a relatively early phase of development, one can expect hybrid versions to first be established before fully continuous production systems emerge. In these systems, the continuous portion may include a perfusion bioreactor integrated with a continuous capture step. As experience grows and new enabling technologies are developed, subsequent downstream unit operations can be converted to continuous and integrated into the system.
4.3. Further optimization of cell culture media
The upstream operational expenses are closely related to the amount and cost of the cell culture media. A strategic need is the development of highly nutritional medium formulation capable of supporting very high cell densities (>50e6 cells/mL) at low volumetric perfusion rates (1-2 bioreactor vol/day). This decreased media consumption, paralleled with low media cost per liter, would result in competitive upstream operational expenses. An obvious prerequisite in the medium optimization efforts is the maintenance of high cell viability and productivity, and consistent product quality during the entire course of the continuous cultivation.
4.4. Global process control
Unit operations are typically designed as autonomous systems equipped with their own local controls. Such local control is sufficient in batch processing, as the unit operations are disconnected and do not need information from each other in order to function properly. While local control is also essential in continuous processing, the integration of unit operations requires global coordination of the entire process flow. Consequently, continuous systems have to be equipped with a second level software control system that supervises and aligns the work of the individual unit operations. Typical oversight functions are coordination of flow rates, event-based control and exception handling. The software and hardware system should provide a high degree of automation, requiring minimal operator involvement. A potential challenge here is that different vendors often use different control systems, which can make such kind of integration difficult. Standards for aligning local and global control will facilitate continuous operation.
4.5. Process Analytical Technology (PAT)
Tightly associated with process automation and control is the need for adequate PAT. Reliable long-term operation of the continuous system can be accomplished if adequate real-time information is available for each unit operation. To a significant extent this has been achieved for bioreactor operation, as demonstrated by the successful multi-month operation of existing commercial perfusion processes. Physical and physico-chemical parameters are reliably monitored in continuous chromatography systems (e.g., UV absorbance, pH, conductivity, flow rate, pressure, etc.) using technologies that are well established in batch processes. What is missing, however, is sufficient amount of real-time information about product quality attributes (activity, aggregation, glycosylation, impurity level, etc.). Development of PAT that addresses this need will be of tremendous importance. Since this is a task of high complexity, reliable on-line sensor solutions are unlikely to emerge in the near term. A more realistic option is the implementation of approaches based on sterile sampling and at-line rapid analysis using established instrumentation. Such systems can be positioned at several strategic points along the process flow, and can work in “quasi real-time”, supplying control systems with the required product quality information. In any case, the progress in this area is expected to be incremental, reflecting the ever improving capability of the analytical technology.
4.6. Single Use Technology
The majority of end users consider the further expansion of capacity from a “facility of the future” standpoint to be based upon single-use closed technology, regardless of the mode of operation (batch or continuous). As the key benefits of single-use, such as low capital cost, low downtime and flexible footprint, are similar to these provided by continuous processing, there is a strong synergy between these two technologies. With improvement in upstream productivity, systems may be smaller, further benefiting single-use technology. Therefore, single use technology is often seen as an enabler for continuous closed processing, including both up- and down-stream operations. While single use technology has made an impressive progress over the last several years, there are still significant needs for alignment between suppliers and end users on key issues, such as GMP regulatory requirements, need for standardization in E&L testing procedures, standards for bag/tubing manifold systems, lack of robust ON/OFF sterile connectors, etc. Addressing these issues will accelerate and expand single use implementation and ultimately reduce the nervousness of continuous processing implementation.
5. Issues to be addressed
As with any new technology, continuous bioprocessing is not yet mature and requires sustained innovation and guided evolution in terms of methodology and technology. Here, we review several high-impact areas that need particular attention.
5.1. Regulatory roadmap
While there is significant experience with continuous perfusion systems, a partial or complete integration between upstream and downstream has not yet been commercially demonstrated. The impressive successes of continuous flow processing in the pharmaceutical industry have attracted the attention of the regulatory authorities, who are increasingly supportive of this new manufacturing paradigm. FDA has recently provided comments about the applicability of continuous manufacture of synthetic drugs with highly encouraging conclusions.[22] As the parallels to biotech are obvious, these discussions provide a framework and path to address potential regulatory hurdles for implementation of the novel continuous bioprocessing paradigm. In order for companies to be sufficiently motivated to move towards continuous technology, it is important that the regulatory path does not impose unrealistic challenges that would counterbalance the discussed technological benefits. As industrial and regulatory experience grows, the path to approval is expected to become simpler and shorter.
Most recently, a presentation focusing specifically on continuous biomanufacturing was delivered by the FDA.[12] The key conclusions were in line with previous guidance in the context of small molecule drugs. The main take home messages are that there is “nothing in regulations or guidance prohibiting continuous manufacturing,” and “continuous manufacturing is fully consistent with FDA’s QbD in which envelopes of performance are seen as part of the control strategy.” There is clarity that lot sizes may be determined by either mass or time. The regulatory expectations for assurance of reliable and predictive processing in a commercial setting are the same for batch and continuous processing.
5.2. Challenges to adoption
According to Reay2 the reasons for the relatively slow penetration of new processing methodologies include, low tolerance of risk, management concerns about implementing new technologies, overinvestment in existing facilities designed for the old principles, the legacy effect of fully depreciated production plants and, not the least, perceived regulatory constraints. While these impediments to change are particularly strong in the field of biopharmaceuticals, where there is a relationship between the process and the product, the evolving competitive business environment and desire to address global markets is incrementally driving the biotech industry towards a tipping point where existing barriers may be counterbalanced by the need for radically improved bioprocessing. In all cases, the success of the introduction of innovative approaches depends not only on sound technical vision, but also on broad support from the entire organization as a matter of corporate strategy.[23],[7]
Well-founded or not, process related concerns include those regarding start-up and shutdown material losses and costs, long validation times, ability to achieve robust process throughput, fears that equipment cleaning may be more difficult or complicated, and the perception that when any unit operation in continuous processing is down for any reason, the whole process is down.[24]
Most importantly, the adoption of continuous processing is facing the challenge of the deeply entrenched “batch technology mindset,” which penetrates organizations at all levels. The implementation of a new technology cannot be a bottom-up initiative, and the complete support of top management is essential. To this end, a strategic vision backed by a convincing business case and solid development scale data needs to be prepared with broad consensus; this is particularly important for the early adopters. Careful selection of the product for the first implementation of a new technology is essential, as concerns about extending the time-to-market may play a critical role in technology selection.
5.3. Supply chain considerations
It is known from other industries, such as food and chemicals, where continuous processing has been applied for a long time, that there is an increased dependence on quality and consistency of raw material in the supply chain. This is essential in order to minimize variance going into the process. If single-use technologies are incorporated into the process, there is greater dependency on technology vendors. In these cases, extra rigor in quality assurance will be required because it takes on additional importance.
Continuous operation also has the potential to positively influence the downstream side of the supply chain, as there is greater flexibility in meeting variable demands from the market since scaling is by time extension and not by the scale of batches. This is further enhanced by the short process cycle time and the opportunity to reduce inventories at hands. Demand may also be met by the mobility of smaller production systems and the opportunity to decentralize production and distribute manufacturing locally closer to markets.
5.4. Impact on business strategy
There is a significant positive impact on the business strategy that is enabled by the increase in flexibility, shortened scale-up and process development time, standardization and reduced capital requirements for an operating plant. The flexibility will permit a more responsive supply chain that is especially important in new and uncertain markets. The lower capital costs may enable smaller companies to integrate manufacturing earlier in their life-cycle and reduce the dependence on CMOs. With smaller plants that can be scaled by time rather than volume, the same facility can be used for clinical material manufacturing and product launch (i.e., multi-purpose facilities). Market growth can be accommodated by longer production cycles or installation of identical parallel process trains. With a standard production platform, the same unit may be used for multiple products, which further increases operational flexibility and cost structure.
5.5 Impact on organizational structure
Accommodation of continuous production will likely require changes in organizational structure. Upstream and downstream distinctions will blur as the bioreactor and chromatography steps become integrated unit operations of one continuous stream. This will have implications on the development and manufacturing organization that will also need to be integrated across the process. These structural modifications will require a change in the culture of the organization. Operational excellence will require compliance under sustained and continuous operation, with an emphasis on process control. Longitudinal data will be collected and new methods for learning will need to emerge.
6. Examples
The potential for, and experience with, continuous processing is illustrated with several examples drawn from recent literature on therapeutic protein manufacturing using mammalian cell culture. The four process architectures (Fig. 1-4) are idealized to illustrate each approach and do not try to capture all the technical details. The examples include three hybrid systems and one fully integrated continuous process. All cases demonstrate the advantages of continuous processing, with the fully continuous system offering the strongest potential.
6.1. Hybrid system: Continuous upstream with batch downstream
The process architecture in Fig. 1 is widely used today for commercial manufacturing of complex/labile proteins, such as enzymes, blood factors and, in some cases, mAbs. Over the last 25 years, several companies, including Genzyme, Bayer, Janssen, BioMarin, Shire, Merck-Serono, Novartis and Pfizer, have practiced perfusion culture. The bioreactor residence time of the product is short, usually <24hrs, which enables the production of unstable proteins with minimal degradation. The harvest is collected in large tanks, and processed downstream in a traditional batch fashion, starting with clarification for cell removal and a capture step. The utilization of filtration based cell retention devices, such as ATF or TFF, enables integration of bioreactor operation and clarification into a single unit process.[25],[26],[19],[27]. Pollock at al.[27] have illustrated how the economic competitiveness of bioprocesses employing perfusion culture depends on cell density increase that is achievable in perfusion systems.
This process configuration allows utilization of small and even mobile bioreactors in the upstream production suite. However, the batch downstream operation still requires large equipment, which minimizes some of the benefit accrued in the continuous upstream design. Nevertheless, perfusion cell culture has been of tremendous use to the biotechnology community because it has enabled the production of unstable proteins, which is difficult or impossible to accomplish with traditional fed-batch technology.
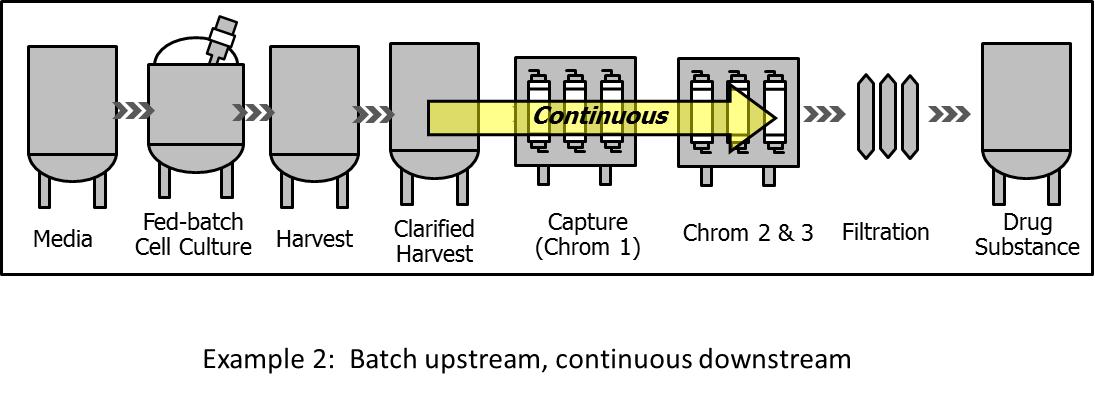
6.2. Hybrid system: Batch upstream with continuous downstream
This process architecture has been recently explored at development and pilot scale (Fig. 2). The cells are cultured in traditional fed-batch mode, while one or more of the downstream unit operations are converted into continuous operation. Various continuous unit operations are being developed, such as precipitation[28], flow-through purification[29], directly coupled chromatography columns without hold vessels in between[30] and viral inactivation[31]. In most of these cases, the continuity of the downstream operation is defined within the limits of a single upstream batch on a mass basis. This mode of operation has been shown to be economically beneficial.[32] For example, the much smaller volume of continuous chromatography columns results in significant savings when expensive chromatography resins are used (e.g., Protein A). Bioprocesses utilizing continuous chromatography for product capture offer significant direct cost savings in clinical material production, which can have a large impact considering the high clinical attrition rates.[32]
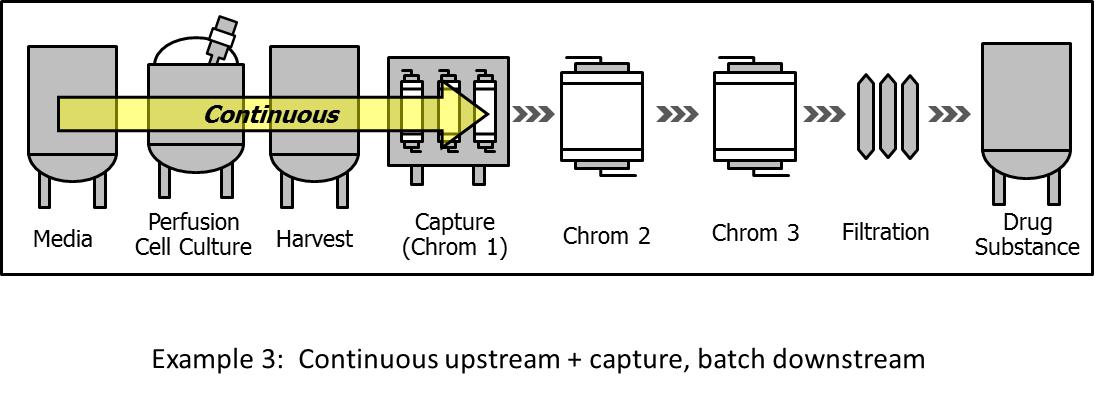
6.3. Hybrid system: Continuous bioreactor and capture followed by batch (post-capture) downstream
In this model, the continuous processing extends downstream of the bioreactor to include the capture step, as shown in Fig. 3. This integration is considered particularly advantageous for two reasons. First, is the elimination of large hold tankage (including clarification unit operations) between the bioreactor and the capture step. Second, the implementation of continuous capture results in 1-2 orders of magnitude reduction in column size and lowers buffer utilization, which is particularly important when the capture step employs columns of large diameter. Various versions of Simulated Moving Bed (SMB) multicolumn systems are being used for the implementation of the continuous capture step. An example of this approach for the production of a recombinant enzyme and mAb is described by Warikoo et al.[33] A 3-column continuous chromatography system was integrated with a high cell density perfusion bioreactor. The system was successfully run for a period of two months, and achieved steady state operation with respect to product quality.
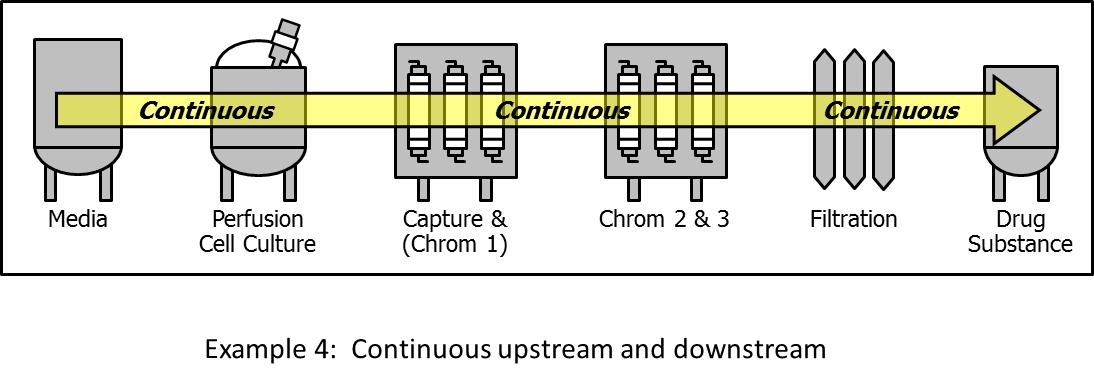
6.4. Fully integrated continuous process
This process configuration consists of fully integrated continuous unit operations for the entire process train (Fig. 4). Development scale examples of such systems have been presented recently.[34],[35] The fully integrated continuous process offers impressive advantages, such as small equipment footprint, low residence and cycle times, and low cost. However, not all unit operations required for the implementation of such a system are commercially available at scale today. Additional efforts are required to establish robust viral inactivation or removal systems that operate continuously at operating scale. The same applies to the UF/DF step at the downstream process. The direct coupling of the individual unit operation requires compatibility of their interfaces, which points to a generic need to focus on interfaces between unit operations. The single-use technology offers advantages in this respect because of the utilization of weldable plastic tubing of various diameters.
7. Recommendations
Continuous processing offers exciting new opportunities for improving the manufacture of biological products. For this technology to be successfully implemented, the joint efforts of industry, academia, regulatory authorities and equipment vendors are needed. Below are a few recommendations that will help to address the existing technical and non-technical challenges:
- Knowledge and learning. The impact of continuous bioprocessing will evolve from the collective action of academic and industrial researchers, via knowledge sharing on systems and their performance as well as ongoing evaluation of innovative ideas. It is recommended that there be a regular forum for bringing together the academic, industrial and regulatory community to review results, propose new technologies, and promote discussions on development and deployment of continuous bioprocessing. Examples of such events are the biannual Integrated Continuous Biomanufacturing Conference (Oct 20-24, 2013, Barcelona) and the International Symposium on Continuous Manufacturing of Pharmaceuticals (May 20-21, 2014; MIT-Cambridge, USA). Workshops between industry and FDA/EMA will also help guide industry during the early phases of development and implementation of this new technology.
- Equipment vendors play a key role in the development and implementation of continuous technology. Industry-vendor forums need to be established to define the requirements for continuous biomanufacturing unit operation (longevity, closed systems, sterility) and the interfaces between them.
- There are several mission critical requirements for production of safe and efficacious biologic products that include viral clearance, in-process testing, supply chain audit, etc. that will be different for batch and continuous bioprocessing. These activities need to be addressed through research in an open forum so that manufacturers and equipment vendors can bring forward the best processes for therapeutic products.
- There is an important role for government in addressing the related issues. Cooperation between FDA and industry to develop a road-map for the implementation of continuous bioprocessing will be highly beneficial. Government funding of related research in academia will accelerate progress in the field. An example is the provision of certain incentives to equipment/instrumentation vendors to develop required continuous unit operations (particularly downstream) and PAT instrumentation.
- Industry-academia collaborations in continuous bioprocessing (such as the Novartis-MIT alliance) will enable sharing of risk and cost, and may be established perhaps in the form of academia-industry consortia to develop, for example, common unit operations and platforms.
8. References
[1] Tanner HA. 1998. Continuous Casting: A Revolution In Steel. Write Stuff Syndicate
[2] Reay D, Ramshaw C, Harvey A. 2008. Process Intensification: Engineering for Efficiency, Sustainability and Flexibility. Butterworth-Heinemann.
[3] Anderson NG. 2001. Practical use of continuous processing in developing and scaling up. Organic process research & Development. 5 (6): 613-621.
[4] Thomas H. 2008. Batch-to-continuous – coming out of age. The Chemical Engineer. 805: 38-40.
[5] Fletcher N. 2010. Turn batch to continuous processing. Manufacturing Chemist. June 2010 24-26.
[6] Laird, T. 2007. Continuous processes in small-scale manufacture. Organic process research & Development. 11 (6) 927.
[7] Utterback, JM. 1994. Mastering the Dynamics of Innovation. Harvard Business School Press.
[8] Bisson W. 2008. Continuous manufacturing – the ultra lean way of manufacturing. ISPE Innovations in process technology for manufacture of APIs and BPCs. Copenhagen, 7-11 April 2008.
[9] Integrated Continuous Biomanufacturing Conference, Oct 20-24, 2013, Barcelona
[10] International Symposium on Continuous Manufacturing of Pharmaceuticals, May 20-21, 2014, MIT-Cambridge, USA
[11] Moore, C. Continuous Manufacturing – FDA Perspective on Submissions and Implementation, 3rd Symposium on Continuous Flow Reactor Technology for Industrial Applications, Lake Como, 2-4 Oct, 2011
[12] Baker, J. 2013. Matching flows: The development of continuous bioprocessing, new initiatives in the approval of bioproducts, and assurance of product quality throughout the product lifecycle. Integrated Continuous Biomanufacturing Conference, Oct 20-24, 2013, Barcelona
[13] Christensen CM. 2000. The Innovator’s Dilemma, New York, NY: HarperBusiness.
[14] Levine, HK. 2010. Disruptive technologies for the future of biomanufacturing. Bio International Convention. Chicago, IL, May 4, 2010.
[15] Badman, C; Trout, B. 2014. Introductory white paper. Achieving Continuous Manufacturing. International Symposium on Continuous Manufacturing of Pharmaceuticals, May 20-21, 2014, MIT-Cambridge, USA
[16] Robinson, D., Chan, C., Lp, C., Tsai, P., Tung, J., Seamans, T., Lenny, A., Lee, D., Irwin, J., Silberklang, M. (1994) Characterization of a recombinant antibody produced in the course of a high yield fed-batch process” Biotechnology and Bioengineering, v. 44 (6)), 727-735.
[17] Reid, C., Tait, A., Baldascini, H., Mohindra, A., Racher, A., Bilsborough, S., Smales, C., Hoare, M. (2010) Rapid Whole Monoclonal Antibody Analysis by Mass Spectrometry: An Ultra Scale-Down Study of the Effect of Harvesting by Centrifugation on the Post-Translational Modification Profile, Biotechnology and Bioengineering, v 107 (1), 85-95
[18] Pacis, E., Yu, M., Autsen, J., Bayer, R., Li, F. (2011) Effects of Cell Culture Conditions on Antibody N-linked Glycosylation - What Affects High Mannose 5 Glycoform, Biotechnology and Bioengineering, v. 108, (10), 2348-2358
[19] Clincke, M-F, Mölleryd, C., Samani, P., Lindskog, E., Fäldt, E., Walsh, K., Chotteau, V. (2013) Very high density of Chinese hamster ovary cells in perfusion by alternating tangential flow or tangential flow filtration in WAVE bioreactor™—part II: Applications for antibody production and cryopreservation. Biotechnology Progress, v. 29 (3), 768-777.
[20] Vogel JH, Nguyen H, Pritschet M, ,Van Wegen R, Konstantinov K. 2002. Continuous Annular Chromatography: General Characterization and Application for the Isolation of Recombinant Protein Drugs. Biotech Bioeng. 80 (5) 559-568.
[21] Napadensky, B., Shinkazh, O., Teella, A., Zydney, A. (2013) Continuous Countercurrent Tangential Chromatography for Monoclonal Antibody Purification, Separation Science and Technology, v. 48 (9), 1289-1297
[22] Godwin F. 2011. Continuous Manufacturing, a Regulatory Perspective. INTERPHEX, New York, March 2011.; FDA, 2011
[23] Shott, I. Roadmap for the Twenty-first Century. Institution of Chemical Engineers, May 2007
[24] Whitford, W. (2013) Single-use Technology Supporting Continuous Biomanufacturing. Integrated Continuous Biomanufacturing Conference, Oct 20-24, 2013, Barcelona
[25] Johnson, T. (2013) Upstream development of high cell density perfusion process for continuous manufacturing. Integrated Continuous Biomanufacturing Conference, Oct 20-24, 2013, Barcelona.
[26] Desai, S. (2013) Continuous and Semi-continuous Cell Culture for Production of Blood Clotting Factors. Integrated Continuous Biomanufacturing Conference, Oct 20-24, 2013, Barcelona.
[27] Pollock, J., Ho, S.V., Farid, S.S. 2013. Fed-batch and perfusion culture processes: operational, economic and environmental feasibility under uncertainty. Biotechnology & Bioengineering, 110(1) 206–219.
[28] Hammerschmidt, N. , Tscheliessing, A., Sommer, R., Helk, B., Jungbauer, A. (2014) Economics of recombinant antibody production processes at various scales: Industry-standard compared to continuous precipitation. Biotechnology Journal. V. 9, 766-775
[29] Xenopoulos, A. (2013) A new, integrated, continuous purification process template for monoclonal antibodies. Integrated Continuous Biomanufacturing Conference, Oct 20-24, 2013, Barcelona.
[30] Landric-Burtin, L. (2013) Simplicity is the key: Continuous purification of monoclonal antibodies, Integrated Continuous Biomanufacturing Conference, Oct 20-24, 2013, Barcelona.
[31] Brower, M. (2013) Platform downstream processes in the age of continuous chromatography: A case study. Integrated Continuous Biomanufacturing Conference, Oct 20-24, 2013, Barcelona.
[32] Pollock, J., Bolton, G., Coffman, J., Ho, S.V., Bracewell, D. G., Farid, S.S. 2013. Optimising the design and operation of semi-continuous affinity chromatography for clinical and commercial manufacture, Journal of Chromatography A, 1284, 17-27.
[33] Warikoo, V., Godawat, R. Brower, K., Jain, S., Cummings, D., Simons, S., Johnson, T., Walther, J., Yu, M., Wright, B., McLarty, J., Karey, K., Hwang, C., Zhou, W., Riske, F., Konstantinov, K. (2012) Integrated Continuous Production of Recombinant Therapeutic Proteins, Biotechnology and Bioengineering. v. 109 (12) 3018-3029.
[34] Godawat, R. (2013) Integrated and Fully Continuous Processing of Recombinant Proteins – From Cell Culture Media to Purified Drug Substance. PREP, July 17, 2013, Boston MA
[35] Daszkowski, T. (2013) Continuous Processing in Biotech Production: An alternative to a modern single use, batch, facility? Integrated Continuous Biomanufacturing Conference, Oct 20-24, 2013, Barcelona.