Link to final published paper
Download this White Paper (revised version)
Download this White Paper (original version)
Download Slides
Control Systems Engineering in Continuous Pharmaceutical Manufacturing

Core Team Members: Allan S. Myerson, Markus Krumme, Moheb Nasr, Hayden Thomas, Richard D. Braatz
Executive Summary
This white paper provides a perspective of the challenges, research needs, and future directions for control systems engineering in continuous pharmaceutical processing. The main motivation for writing this paper is to facilitate the development and deployment of control systems technologies so as to ensure quality of the drug product. Although the main focus is on small-molecule pharmaceutical products, most of the same statements apply to biological drug products.
An introduction to continuous manufacturing and control systems is followed by a discussion of the current status and technical needs in process monitoring and control, systems integration, and risk analysis. Some key points are that: (1) the desired objective in continuous manufacturing should be the satisfaction of all critical quality attributes, not for all variables to operate at steady-state values; (2) the design of start-up and shutdown procedures can significantly affect the economic operation of a continuous manufacturing process; (3) the traceability of material as it moves through the manufacturing facility is an important consideration that can at least in part be addressed using residence time distributions; and (4) the control systems technologies must assure quality in the presence of disturbances, dynamics, uncertainties, nonlinearities, and constraints. Direct measurement, first-principles and empirical model-based predictions, and design space approaches are described for ensuring that CQA specifications are met.
Ways are discussed for universities, regulatory bodies, and industry to facilitate working around or through barriers to the development of control systems engineering technologies for continuous drug manufacturing. Industry and regulatory bodies should work with federal agencies to create federal funding mechanisms to attract faculty to this area. Universities should hire faculty interested in developing first-principles models and control systems technologies for drug manufacturing that are easily transportable to industry. Industry can facilitate the move to continuous manufacturing by working with universities on the conception of new continuous pharmaceutical manufacturing process unit operations that have the potential to make major improvements in product quality, controllability, or reduced capital and/or operating costs.
Regulatory bodies should ensure that: (1) regulations and regulatory practices promote, and do not derail, the development and implementation of continuous manufacturing and control systems engineering approaches; (2) the individuals who approve specific regulatory filings are sufficiently trained to make good decisions regarding control systems approaches; (3) provide regulatory clarity and eliminate/reduce regulatory risks; (4) financially support the development of high quality training materials for use of undergraduate students, graduate students, industrial employees, and regulatory staff; (5) enhance the training of their own technical staff by financially supporting joint research projects with universities in the development of continuous pharmaceutical manufacturing processes and the associated control systems engineering theory, numerical algorithms, and software; and (6) strongly encourage the federal agencies that support research to fund these research areas.
Keywords: control systems, manufacturing, process monitoring, quality by design, process analytical technology, systems integration, design space, feedback control, plant-wide control
1. Introduction to Continuous Manufacturing and Control Systems
In recent years, pharmaceutical companies, federal agencies, and some universities have become interested in the development of technologies for the continuous manufacturing of drug products. In addition to benefits in terms of providing improved quality of drug product from translating existing batch processes directly to continuous, many examples have been published in which orders of magnitude improvements in process efficiency or controllability have been demonstrated. Many of the driving applications have involved the invention of very fast or high pressure organic chemistry pathways that can only be effectively operated in small-scale continuous-flow reactors.[1,2,3,4] Very fast chemical reactions typically cannot be operated in a batch due to the poor spatial homogeneity in batch vessels and the inability to transfer heat at a rate that is high enough to avoid the generation of undesirable by-products or thermal degradation of the desired drug compound. Another set of driving applications that have been used to manufacture commercial drug products have applied continuous-flow mixers to produce drug crystals with very narrow size distributions (see Figure 1), with a degree of size uniformity that cannot be achieved in a batch due to spatial inhomogeneity.[5]
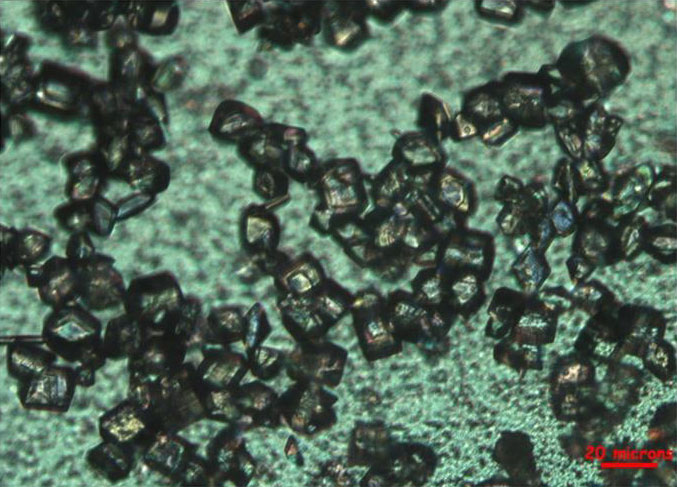
Fig. 1. Crystals produced in continuous-flow by a dual-impinging jet mixer.
Continuous processes require control systems to ensure that the products are of high quality. Continuous pharmaceutical manufacturing processes can range from chemistries that involve only fluids, where substandard fluid can often be mixed with above-standard fluid to produce a fluid mixture that satisfies specifications, to solids whose specifications cannot be met in this manner. For the pharmaceutical industry, the control systems must provide a higher assurance of consistent product quality than what is required in most processes in the chemical, oil refining, and petrochemical industries. The objective of this paper is to provide a perspective of the current state of control systems engineering in the pharmaceutical industry and discusses the technical needs, challenges, and future research directions to facilitate the deployment of control systems technologies so as to ensure persistent quality of the drug product.
As an introduction, it is useful to provide a high-level description of the needs for control systems in the pharmaceutical industry. Very few manufacturing operators in the industry have any process automation or control expertise, so it is especially important that the control interfaces be user-friendly, while providing accurate and consistent control of the manufacturing facility and enabling the user to monitor and interface with the facility in a safe and efficient manner. The control system should provide only the necessary functionality, without having an overly complicated human-machine interface (HMI), to allow the operator to routinely verify that process parameters are within normal operating ranges and acceptance limits and that, when alerts and alarms are triggered, the necessary actions can be determined quickly, without scrolling through multiple views. System architectural design should not involve unnecessarily complicated and time-consuming development to customize the software to the particular plant, to allow for easy maintenance.
Other considerations required of the overall system are the maximization of the uptime vs. downtime ratio, minimization of maintenance requirements, inclusion of performance diagnostics, and capability of future expansion. At first glance these requirements may seem formidable, but much of the technical framework for such control systems already exists in other industries, such as oil refining, chemicals, and petrochemicals where such features are business as usual. As stated above, there are compelling differences, due to pharmaceutical products having a much higher requirement for continual assurance of product quality during processing than for non-pharmaceutical flow systems, but enough of the technical framework is in place that the control systems engineering can develop at a much faster pace for the continuous-flow manufacture of drug products than the fifty plus years it took for control systems engineering to develop in other industries.
The remainder of this paper begins with a discussion of the current needs for control systems engineering in the continuous manufacture of pharmaceutical products, and the technical barriers to addressing these needs. Then what industry, regulatory bodies, and universities can do to facilitate working around or through these barriers to develop control systems engineering technologies for continuous manufacturing is discussed. This discussion is followed by a description of existing and future control systems engineering technologies that could be of the most benefit to continuous pharmaceutical manufacturing, and a discussion of research directions that should be pursued to develop these technologies.
2. Current Status and Needs
2.1. Steady-state and Dynamics in Continuous Manufacturing
In the chemical engineering field, steady-state refers to operations in which none of the variables in the system vary as a function of time. For a manufacturing facility, steady-state refers to all process variables – including pressures, temperatures, compositions, tank levels, and flow rates – and all variables associated with the control system, such as setpoints, measured variables, and manipulated variables. Steady-state is sometimes a useful idealization, but the term is much more commonly used by those who are not control engineers rather than by those who are. The reason for this difference in usage is that control engineers know that any real industrial system is never operating at steady-state due to disturbances, such as pressure fluctuations, variations in the temperature of the surroundings that affect that rate of heat transfer to the system, and its variations in the compositions of the chemical feedstocks. Furthermore, many unit operations, such as adsorption, ion exchange, and chromatography columns cannot be operated under steady-state conditions and are typically operated at the industrial scale with multiple columns with time-varying flows that switch between the columns. Additionally, many variables have no incentive for being held at a constant value, with one common example being the level of a tank. The level of a tank is not a product quality specification, and so a common strategy used by process control systems is to actively vary a tank level to produce smaller time variations in a variable that directs impacts product quality.[6] It is also common for a drug product to have an allowed range of critical quality attributes (CQA), with no clear benefit for being exactly at setpoint values. For example, the concentration of an impurity in the drug product typically has an upper boundary with no penalty for further reduction of the impurity. In this case, it is often possible to improve process efficiency or enable one set of CQAs to stay within its specifications by allowing another CQA to vary while staying within its acceptance limits.[7] Certainly meeting all of the CQA specifications is more desirable than violating specifications due to a perceived desire to try to force all of the CQAs to be at some nominal “steady-state” values.
Furthermore, the control system is specifically designed to vary one set of variables, commonly referred to as manipulated variables, as degrees of freedom available for control, to ensure that all of the CQAs are within specifications. For the control system to be able to fulfil its intended purpose in suppressing the effects of disturbances on the CQAs, the manipulated variables will continuously vary over time rather than have steady-state values. Forcing the degrees of freedom manipulated by the control system to steady-state values would make it impossible for the control system to serve its intended purpose of ensuring product quality, so operating a process at steady-state is undesirable. What is important to consumers is ensuring that the states of the manufacturing system are under control, that is, that drug product quality is ensured – not whether the manufacturing system is operating at steady state.
For these reasons, expert process control engineers are not fixated on trying to achieve “steady” or “steady-state” operations. For manufacturing facilities in which such unit operations are not present, and when the product quality specifications are fixed, the process control system is usually designed to purposely vary the manipulated variables over time, based on the time-varying measured variables to try to achieve reduced variations in the product quality variables. In other words, the goal of the control system is to purposely vary one set of variables over time so as to create small variations in other variables over time.
Minimizing the time to steady-state should not be the main objective of continuous manufacturing; the objective should be to minimize the production of out-of-spec drug product.
An important consideration in the design of a continuous-flow process is the residence time distribution (RTD), which is the distribution function for the amount of time that a fluid element spends inside the vessel. Plug flow is one idealized RTD, in which there is no back-mixing and all fluid elements spend exactly the same amount of time in the vessel. The same notions apply to processes that have both fluids and particles. The reality is that there is always some intermixing of fluid elements, but many continuous-flow designs are specifically designed to approach plug flow as closely as possible. Plug flow operations create time delays in the process dynamics that can reduce the closed-loop performance achievable by the control system. The effects of such time delays can often be mitigated by using feedforward of measured disturbances to the control system or by having intermediate placement of sensors/analytics within the unit operation. Having such real-time detection, in addition to an understanding of RTD for the unit operations, affords the ability to track materials through the process to determine acceptability and the potential to isolate material in the case of out-of-spec material.
The tracking of product through the process and the design of the associated control systems becomes more complicated once multiple unit operations of different system dynamics become connected.
A continuous-flow process will need time to start up and shut down, and can have other perturbations in its dynamics as discussed above. A rough rule of thumb is that a well-controlled manufacturing facility should take about five times the mean residence time to start up (approximately reach quasi-steady operations), provided that the equipment has employed warm starts, which means warming up or pre-feeding intermediate equipment with chemical of intermediate compositions. The time required for individual unit operations to reach quasi-steady operation can vary by many orders of magnitude, from less than one second to hours. As such, the start-up time is a strong function of the unit operations that take the longest time to reach quasi-steady conditions. This principle applies for single unit operations as well as for sequences of unit operations. The relevant RTD to estimate the expected start-up time is in all cases the RTD between entry point and exit point of the sequence of unit operations in question. The mean residence time serves as a first level estimator in this rule of thumb but it needs to be understood that the RTD may be very wide in sequences of multiple unit operations, in fact, so wide that direct experimental verification may barely be possible. The RTD also tends to become wider as the more recycle loops are designed into the manufacturing process. Recycle loops, however, are often attractive for their ability to improve transformation qualities, such as yields or certain quality attributes. The practical consequence, in these cases, is that the “area of influence” of a disturbance on the time axis can be extremely wide, or in other words, the longest residence time or system dynamic can be extremely slow. Controls that are effective in maintaining closed-loop stability should be placed into the lowest level loops, with upper level loops designed so that the product satisfies CQAs as quickly as possible while maintaining system stability. The control loops for each unit operation need to be well tuned before the upper level control loops are tuned, and their effects on the manufacturing facility need to be taken into account when determining its overall RTD.
The time required to shut down a manufacturing facility is much less than the time required for start-up, typically only about two times the mean residence time. At any rate, both of these times are long enough that it is highly desirable for the control system for any continuous pharmaceutical manufacturing process to be designed in such a manner that the drug product meets CQA specifications during the start-up and shutdown phases, not just during quasi-steady operations. A question for research is how to design optimal start-up and shutdown procedures for both particular unit operations and for continuous pharmaceutical manufacturing facilities, such as having segregation points throughout the process to rapidly remove “transition” material. As suggested above, this optimization should be focused on minimizing off-spec product rather than minimizing the time to quasi-steady operations.
When designing control systems, it is important to consider the capability of each piece of equipment and maintain a systems point of view of the various unit operations and their capacities. Understanding the individual unit operations and their interactions is critical to the design of any well-designed process control system.
2.2. Process Monitoring and Control
In addition to the well understood importance of monitoring and maintaining the quality of the incoming materials to a pharmaceutical manufacturing facility, it is also important to maintain the in-process material in a state that ensures that the final product will be of a consistent uniform character and quality within specified limits. Appropriate acceptance criteria need to be set, and the processes need to be understood, which can be characterized in terms of the operating design space. The in-process controls consist of analytical measurements, manipulated variables (e.g., pump flow rates), and the feedback controller. In Quality-by-Design (QbD) terminology, critical process parameters (CPPs) are process parameters critical for controlling the downstream product quality, with respect to the specified incoming material attributes or in-process material attributes from an upstream process.[8] It is important to distinguish feedforward control, in which manipulations are made in response to measurements of disturbances, from feedback control, in which manipulations are made in response to measurements of variables that need to be controlled. A continuous process can be controlled in a truly dynamic fashion whereby downstream process parameters can be manipulated in response to measured upstream disturbances to maintain final product quality and in response to local real-time measurements of product quality. Using both feedforward and feedback control to respond in real time to disturbances throughout the multiple unit operations is a hallmark of continuous manufacturing.
The closed-loop dynamic effects of feedforward and feedback control are very different and need to be respected during the design of the control system.[9] Also key to systematic controls systems design is the characterization of the disturbances and the propagation of the effects of the disturbances as well as the manipulated variables on the controlled and measured variables. Other important information needed for control design is a defined sampling plan and frequency (the cycle time for analytical measurements) and the interaction between process unit operations. All of this information is needed to systematically design a plant-wide operational control strategy.[6]
A mathematical model is said to be based on first-principles if constructed based on material, molar, energy, and momentum conservation equations; chemical reaction networks; thermodynamics; and transport flux equations such as Fick’s Law of Diffusion, Fourier’s Law of Heat Conduction, and Newton’s Law of Viscosity. One approach to the design of a plant-wide control strategy is to develop and validate first-principles models for each unit operation, in isolation, and then place each of these unit operation models into a common platform for simulation of the entire manufacturing facility that is then used for the design and evaluation of a plant-wide control strategy. This approach was implemented successfully for the continuous manufacturing of aliskiren.[6,10,11,12] Realistic models of uncertainties and disturbances were implemented in simulation to design and evaluate the effectiveness of the plant-wide control system before the construction of the continuous manufacturing facility was completed. Plant-wide simulations indicated that all product purity specifications would be satisfied once all of the control loops were closed, which was observed during the operation of the facility.[10] This application of model-based control design allows the reduction of the risk of running into unexpected operational or quality control problems once the manufacturing facility is brought online, and to evaluate whether the control system with the existing in-process process analytical technology/on-line monitoring can achieve real-time release instead of or in concert with end-product testing.
Such first-principles models, or well-characterized empirical or semi-empirical models, can be used to assess not only when the current product quality is in-spec and that the overall process operations are under control, but also to predict that the drug product will remain in-spec into the future. In the aliskiren continuous manufacturing plant, for example, the first-principles simulation model for the plant was continuously updated during operation so as to predict the future values for all variables in the model. This information can be used for evaluation of the state of control of the system and to quickly locate equipment faults or unexpected operational problems, by comparing the values of measured variables with variables predicted by the simulation model.
Only profound knowledge of the system dynamics can be the basis for establishing the accurate and relevant description of the process conditions that have been in place for a particular unit dose of material.
A system also needs to be in place to track material as it moves through the manufacturing facility, which is naturally handled by a combination of measurements and RTDs for the individual unit operations, and the equipment and process for segregating non-conforming material. This material traceability aspect is absolutely key for a sustainable pharmaceutical production facility, as the “area of influence” of a disturbance needs to be clearly understood and on a routine basis provides the means to derive the data set of process variables that is in place to transform the material stream in its way through the facility. As a result of the RTD concept discussed above, it becomes evident that the process conditions in a sequence of unit operations that describe the transformation of a very specific chunk of material travelling along the process chain are not simultaneous, but need to reflect the RTD or at least the mean residence time schedule of the entire network. Only profound knowledge of the system dynamics can serve as the basis for establishing the accurate and relevant description of the process conditions that have been in place for a particular unit dose of material. At the end of the process chain, a process profile (“batch” record) needs to serve as the proof of the process having been in spec, when the specific material that is intended to be released against a specification is subjected to a release decision.
The RTD “schedule” of the sequence in realistic systems may be so broad that experimental verification is not feasible and simulation is the best that can be achieved. Some continuous pharmaceutical manufacturing plant designs inherently have a wide RTD, especially for systems with recycles.
Some continuous pharmaceutical unit operations are inherently easier to control than others, even for unit operations of a particular type such as crystallization. As such, continuous pharmaceutical unit operations should be designed, when possible, so that the exiting materials are high quality without requiring unnecessarily complicated associated control systems. Much of the product quality should be achieved by designing an effective process at the design stage and supplemented, as needed, by additional in-process controls, monitoring, and end-product testing. The control system should not be treated as a Band-Aid for a poorly designed process. For example, an efficient and direct route to control of impurities is to design an organic synthesis route and chemical reactor so that impurities are not generated, which also reduces the complexity and demands of downstream separations processes as well as simplifying the control system design.[13] As another example, a modest change in tank configurations can greatly suppress the effects of disturbances on the exiting material attributes and their effect on downstream unit operations.[14] A research need is to develop both control strategies and design methods for specific new unit operations for continuous pharmaceutical manufacturing.
2.3. Systems Integration
The control systems for the individual unit operations are coupled to a higher-level control layer, typically called the supervisory control layer. Supervisory control manages the flow rates as streams move through the manufacturing process, and manages impurities to ensure that any increases of impurities in upstream unit operations do not become so large that downstream processes cannot handle them, or have insufficient flexibility to deal with other disturbances. The supervisory control layer sends setpoints to the lower level controllers, with the main design criteria typically being to maintain product quality, ensure that there are no production rate mismatches, and that there are no operational problems due to recycle loops.[6] The lower level control loops usually employ real-time in-process measurements whereas supervisory control systems operate on slower time scales and can be updated with some analytical laboratory outputs. The pharmaceutical industry can work within the existing framework of automation standards for rapid deployment and system maintenance and expansion, which was demonstrated for a continuous manufacturing facility for the production of aliskiren.[10]
The overall control system is designed to have a separation of time scales between the supervisory level (slow) and unit operations level (fast) allowing the operator to either focus on operations on the plant-wide level or the single unit operations level. Having a supervisory level in place allows the determination of setpoints to send to the unit operation level to achieve a specified overall production rate while satisfying all of the CQA specifications, enabling the implementation of operation of strategies such as demand-pull, in which inventories are reduced by focusing on the product demand rather than on forecasting.[15]
Process Analytical Technology (PAT) is “A system for designing, analyzing, and controlling manufacturing through timely measurements (i.e., during processing) of critical quality and performance attributes of raw and in-process materials and processes with the goal of ensuring final product quality.”[16] There are multiple approaches to ensuring that any particular CQA specification is met:
- Direct measurement of the CQA,[16,17,18]
- Prediction of the CQA based on a first-principles model that is fed measurements of related variables and is running in parallel with operations,[10,11]
- Prediction of the CQA based on an empirical or semi-empirical model (e.g., response surface map, chemometrics model) that is fed measurements of other variables,[19,20,21] and
- Operation of the CPPs to lie within a design space, that is, some specified set shown in offline studies to provide assurance.[8,9]
In terms of single approaches, approaches ranked higher in the list are the most preferred for assurance that the CQAs as within specification. Further quality assurance can be obtained by redundancy, either within a single approach such as by using multiple sensors in Approach 1, preferably using different measurement principles, or by implementing multiple approaches in parallel.
The plant control software should have real-time display of CPPs, analytical measures, model fits, and trending data. The software should have different user control levels, such as operator, engineer, and administrator, with established standard rules on resolving any issues that arise during operations. All data should be directed to a database for archiving, with the ability to view data online, view trends, and produce plots. The database should be searchable and exportable for the development and maintenance of process models. Concise reporting should be available for real-time release decisions, namely, batch summaries, user actions, alarms, and excursions. Several commercial software packages are available for implementing these functions, including by such companies as AspenTech, Siemens, and OSIsoft. A research need is the development of systems integration methods that respect the higher quality assurance required in the pharmaceutical industry.
2.4. Disturbances, Nonlinearities, Constraints, Uncertainties, and Risk
Section 1 discussed how the level of product quality assurance is much higher for a pharmaceutical product than for most products in the chemical, petrochemical, and oil refining (CPOR) industries for which control systems engineering is previously established. Related to this observation is that the concept of design space is undeveloped in the CPOR industries. Systematic approaches are needed for understanding the integration of design spaces and quality assurance with the design of the overall plant-wide control strategy and the design/tuning of the control systems for each unit operation to take into account disturbances, nonlinearities, dynamics, constraints, and uncertainties.
It is important to be able to quantify the technical risks of failures or delays that occur anywhere in process development.
A key component when making a decision when selecting between competing pharmaceutical technologies is to reduce risk. One form of risk is regulatory risk, whose strategies for reduction are discussed in some detail in Section 3. Another form of risk is technical risk, which is the risk that a particular technology will fail to translate into a robust reliable unit operation during some stage of process development. The greatest financial penalties typically occur for failures or delays that arise during scale-up. Many of the technologies for continuous pharmaceutical manufacturing have reduced scale up of dimensions compared to batch, which reduces scale-up risk, but there is always an inherent risk in introducing any new technology, which is risk associated with uncertainties on how the process will operate. The uptake of continuous manufacturing into companies will be limited unless there are better ways to assess the risk associated with using one of the new technologies.
3. Challenges
3.1. What Can Universities Do?
The main technical challenge in supporting the development of mechanistic understanding down to the unit operations level, and producing focused studies in process modelling, control systems, statistical process control, and automation engineering specific to the needs of the pharmaceutical industry at universities, is the lack of interest of most industrial pharmacy, pharmaceutical engineering, and chemical engineering (IPPECE) departments. Federal support for pharmaceutical manufacturing research has been very low in most countries, which is why most research in this area has been supported by companies, and why IPPECE departments are overwhelmingly focused on alternative areas such as biomedical research in which >$1M grants are typical. IPPECE departments would be willing to hire more faculty to do research in continuous pharmaceutical engineering, and the associated control systems engineering, if industry and regulatory bodies work with federal agencies to create federal funding mechanisms that are competitive with biomedical engineering (e.g., a “National Institute of Pharmaceutical Manufacturing”).
On a technical level, universities should invest in building bridges between algorithm development, and verification of performance, to real-world applicable codes and software objects. For this to happen, a universal control platform might be conceived, which is based on a standardized system architecture that is significant enough to support the necessary control algorithms, as well as the practical usability requirements for process experts that are not necessarily highly skilled control engineers. The control platform would support the implementation of first-principles models for making real-time predictions, methods for the analysis of the effects of disturbances and uncertainties on model predictions, control systems designed for specific classes of unit operations, and plant-wide control systems. Although this activity could be seen as an industrial activity, industrial implementation of such a system quickly runs into the problem of the proprietary nature of the code platform and hence the limited availability for the end-use industry. Firms that might have a substantial interest in developing such a control platform, and have the necessary technical skills to do so, would be highly motivated in limiting the access through a most likely expensive licensing model. Such limits would significantly constrain early algorithm adoption in an already risky disruptive technology implementation like continuous manufacturing, by requiring the shouldering of additional costs and risks. Highly successful models to implement new technology platforms of a similar ground-breaking nature can be found in the information technology industry, where operating systems such as Unix and Linux have been conceived and substantially developed at universities and later refined and implemented in various industries in commercial business models. In cases where the entry port was developed in academic environments, partially with industrial support, numerous examples can be found of such platforms that have been disruptive.
3.2. What Can Industry Do?
The main challenge to industry is cultural. The pharmaceutical industry needs to promote the development of a deep understanding in utilizing newer continuous manufacturing technologies, both within individual companies and in interactions with universities. Industry needs to develop control systems based on that process understanding, on sound engineering principles, and on practices used by other industry sectors. Lastly, industry needs to show a willingness to “make the switch” to continuous manufacturing.
Industry can facilitate the move to continuous manufacturing by working with universities on the conception of new continuous pharmaceutical manufacturing process unit operations that have the potential to make major improvements in product quality, controllability, or reduced capital and/or operating costs. For the translation from universities to companies to be successful, these interactions need to include the development of process models and control systems engineering theory and associated numerical algorithms and software for specific unit operations as well as for whole plants that ensure that every drug product satisfies the CQA specifications. Industry can encourage governments and regulatory bodies to financially support research in the above areas, as the industry can prove the relevance and impact of the solution to the economy and the penetration into our societies.
Industry can facilitate the move to continuous manufacturing by working with universities on the conception of new continuous pharmaceutical manufacturing process unit operations that have the potential to make major improvements in product quality, controllability, or reduced capital and/or operating costs.
As discussed in Section 3.1, universities and industries need to work together in order to be truly disruptive. Industry needs to distinguish between solution providers and the end-customer industry. A pharmaceutical company has no major interest in developing sophisticated technical solutions in a very specialized technical area. The main business model is the development and manufacture of drugs and the necessity of technical tools, like sophisticated control systems, is clearly not the core interest. Such technical tools may be beneficial or even desirable, but certainly will not deliver a competitive advantage in the core business. The scenario for companies whose core business is control is different, as the topic is their main business objective. If, however, a purely commercial business model is applied for the development, then the initial hurdle will be high for the relatively limited number of pharmaceutical companies with the understanding of the value of such a system and hence the adoption low or at least slow, which would be counter to a fast and convincing development of the system – such a scenario just does not amortize the development cost of the system fast enough. The end result is that the control industry would be slow in adopting the pharmaceutically relevant process modelling and control systems developed at universities into their systems, which are much broader in scope than just the pharmaceutical industry.
A solution that maybe worth considering is the concept of academia and industry joining forces in defining an open-source architecture for controls that allows academia to develop and implement the best algorithms on a platform basis, that is accessible to the public under an open license model and hence offers sufficient basis for control companies for specific but compatible implementations amongst different companies, offers end customers like the pharmaceutical industry a solution of wide applicability, and helps the standardization of the implementations. Such an approach also provides a basis for the unified education of skilled process control engineers that would help to spread the technical basis and foster implementation on a broad basis.
The pharmaceutical industry as the end customer can, and must, participate in such an open-source-open-collaboration initiative, as they have the practical problems and need to direct the work towards the problems of greatest practical relevance, after the architecture is put in place. An effort of this magnitude would certainly not be a short-term initiative and would need to be funded by long-term commitments and supported by a long-term strategy with adequately freed up internal resources as well.
3.3. What Can Regulatory Bodies Do?
The main challenge associated with regulatory bodies is to ensure that regulations and regulatory practices promote, and do not derail, the development and implementation of continuous manufacturing and control systems engineering approaches. Regulatory bodies can work closely with the pharmaceutical industry and universities to realize continuous manufacturing. Two things that need to change are the mind-set and regulatory processes to adopt modern innovations based on continuous manufacturing and sound systems engineering principles. Regulatory bodies need to ensure that the individuals who approve specific regulatory filings are sufficiently trained to make good decisions regarding control systems approaches, while not micromanaging the control systems implementations to the point of forcing low productivity or increasing regulatory risks. The key need is to provide regulatory clarity and to eliminate/reduce regulatory risks. [A guideline specific to continuous manufacturing may be counterproductive, but it would be useful for regulatory bodies to serve as a guide to expectations on a case-by-case basis with companies.]
Regulatory bodies should ensure that regulations and regulatory practices promote, and do not derail, the development and implementation of continuous manufacturing and control systems engineering approaches.
Regulatory bodies have a challenge in terms of training, and should financially support the development of high quality training materials in control systems engineering for continuous pharmaceutical manufacturing processes for use of undergraduate students, graduate students, industrial employees, and regulatory staff. Regulatory bodies can enhance the training of their own technical staff by financially supporting joint research projects with universities in the development of continuous pharmaceutical manufacturing processes and the associated control systems engineering theory, numerical algorithms, and software. Regulatory bodies can increase the current very low levels of federal funding for continuous pharmaceutical manufacturing research in most countries, by strongly encouraging the federal agencies that support research to fund these areas.
4. How to Meet the Challenges, Including Future Technologies
Section 2 describes many of the technical needs for control systems engineering in continuous pharmaceutical manufacturing, which included theory, algorithms, and software for:
- The design of optimal start-up and shutdown procedures (Section 2.1).
- The traceability of material as it moves through the manufacturing facility (Sections 2.1 and 2.2).
- The design of process monitoring and control systems that collectively provide high quality assurance (Section 2.2).
- Control strategies and design methods for specific new unit operations (Section 2.2).
- Development of systems integration and data analysis methods that respect the higher quality assurance required in the pharmaceutical industry (Section 2.3).
- Understanding of the integration of design spaces and quality assurance with the design of the overall plant-wide control strategy (Section 2.4).
- The design/tuning of the control systems for each unit operation to take into account disturbances, nonlinearities, dynamics, constraints, and uncertainties (Section 2.4).
- The quantification of the technical risks of failures or delays that occur anywhere in process development (Section 2.4).
In the last decade or so, some efforts have been published that have started to address many of the above technical needs.[22,23] Many of the most closely related past systems engineering methodologies have been applied in microelectronics manufacturing, which share with pharmaceutical manufacturing the inability to obtain acceptable quality by mixing fluids of varying composition as often seen in the chemical, petrochemical, and oil refining industries. The control systems theory needed to develop computationally efficient numerical algorithms that would go into software is well developed for some of the above problems and not for others. Most of the numerical algorithms have limitations in functionality, robustness, and/or computational efficiency, and very few publications demonstrate implementations to continuous pharmaceutical processes. Easy-to-use software has been lacking for implementing the most flexible and computationally efficient of the existing numerical algorithms.
The least developed aspect of the above technical needs concerns the management of technical risk. The quantification of risk requires the quantification of both uncertainties (i.e., model uncertainties, feedstock variations, disturbances) and the propagation of the effects of those uncertainties onto the CQAs. This quantification requires more powerful methods for the quantification of uncertainties from experimental data and for the quantification/assessment of risk throughout the development of a manufacturing process. The design of experiments (DoE) specifically to minimize the overall technical risk, rather than by fractional factorial design or alternative DoE methods currently applied in the chemical and pharmaceutical industries, would enable the design of experiments to produce data most closely aligned with the overall needs of process development. Such analysis would enable the generation of design spaces for continuous pharmaceutical manufacturing processes with a minimum cost for experimentation and with taking scale-up into account. Control systems technology is also needed for the quantitative incorporation of risk into the design of plants and individual unit operations, control systems design, and process scheduling and planning.
References
[1] Patricia Van Arnum. 2013. Advancing flow chemistry in API manufacturing – Continuous flow chemistry offers potential for greater control, improved safety and environmental profiles, and efficient chemical transformations. Pharmaceutical Technology, 37(4):78-82.
[2] Zhenqi Shi, Nikolay Zaborenko, and David E. Reed. 2013. Latent variables-based process modeling of a continuous hydrogenation reaction in API synthesis of small molecules. Journal of Pharmaceutical Innovation. 8(1):1-10.
[3] Peter Poechlauer, Juan Colberg, Elizabeth Fisher, Michael Jansen, Martin D. Johnson, Stefan G. Koenig, Michael Lawler, Thomas Laporte, Julie Manley, Benjamin Martin, and Anne O’Kearney-McMullan. 2013. Pharmaceutical roundtable study demonstrates the value of continuous manufacturing in the design of greener processes. Organic Process Research & Development, 17(12):1472-1478.
[4] D. M. Roberge, L. Ducry, N. Bieler, P. Cretton, and B. Zimmermann. 2005. Microreactor technology: A revolution for the fine chemical and pharmaceutical industries? Chemical Engineering & Technology, 28(3):318-323.
[5] M. Jiang, M. Fujiwara, M. H. Wong, Z. Zhu, J. Zhang, L. Zhou, K. Wang, A. N. Ford, T. Si, L. M. Hasenberg, and R. D. Braatz. 2012. Towards achieving a flattop crystal size distribution by continuous seeding and controlled growth. Chemical Engineering Science, 77:2-9.
[6] R. Lakerveld, B. Benyahia, R. D. Braatz, and P. I. Barton. 2013. Model-based design of a plant-wide control strategy for a continuous pharmaceutical plant, AIChE Journal, 59:3671-3685.
[7] Ali Mesbah, Ashlee N. Ford Versypt, Xiaoxiang Zhu, and Richard D. Braatz. 2014. Nonlinear model-based control of thin-film drying for continuous pharmaceutical manufacturing. Industrial Engineering & Chemistry Research, 53:7447-7460.
[8] Pharmaceutical Development Q8(R2). International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use, ICH Harmonised Tripartite Guideline, August 2009.
[9] M. Kishida and R. D. Braatz. 2014. A model-based approach for the construction of design spaces in quality-by-design. 8:1513-1518. A skewed structured singular value based approach for the construction of design spaces: theory and applications. IET Control Theory & Applications, in press. DOI10.1049/iet-cta.2013.0607
[10] Richard Lakerveld, Brahim Benyahia, Patrick L. Heider, Haitao Zhang, Aaron Wolfe, Chris Testa, Sean Ogden, Devin R. Hersey, Salvatore Mascia, James M. B. Evans, Richard D. Braatz, and Paul I. Barton. The application of an automated control strategy for an integrated continuous pharmaceutical pilot plant. Organic Process Research & Development, in press.
[11] S. Mascia, P. L. Heider, H. Zhang, R. Lakerveld, B. Benyahia, P. I. Barton, R. D. Braatz, C. L. Cooney, J. M. B. Evans, T. F. Jamison, K. F. Jensen, A. S. Myerson, and B. L. Trout. 2013. End-to-end continuous manufacturing of pharmaceuticals: Integrated synthesis, purification, and final dosage formation. Angewandte Chemie, 52(47):12359-12363.
[12] H. T. Zhang, R. Lakerveld, P. L. Heider, M. Y. Tao, M. Su, C. J. Testa, A. N. D'Antonio, P. I. Barton, R. D Braatz, B. L. Trout, A. S. Myerson, K. F. Jensen, and J. M. B. Evans. 2014. Application of continuous crystallization in an integrated continuous pharmaceutical pilot plant. Crystal Growth & Design, 14(5):2148-2157.
[13] David Elder, Kevin L. Facchine, Jeffrey N. Levy, Rodney Parsons, David Ridge, Lesley Semo, and Andrew Teasdale. 2013. An approach to control strategies for sulfonate ester formation in pharmaceutical manufacturing based on recent scientific understanding. Organic Process Research & Development, 16(11):1707-1710.
[14] R. Lakerveld, B. Benyahia, P. L. Heider, H. Zhang, R. D. Braatz, and P. I. Barton. 2013. Averaging level control to reduce off-spec material in a continuous pharmaceutical pilot plant. Processes, 1(3):330-348.
[15] John R. Costanza. 1996. The Quantum Leap in Speed to Market. Englewood, Colorado. John Costanza Institute of Technology, Inc. 352 pages.
[16] Guidance for Industry: PAT – A Framework for Innovative Pharmaceutical Development, Manufacturing, and Quality Assurance, Food and Drug Administration, Rockville, Maryland, September 2004.
[17] P. R. Wahl, D. Treffer, S. Mohr, E. Roblegg, G. Koscher, and J. G. Khinast. 2013. Inline monitoring and a PAT strategy for pharmaceutical hot-melt extrusion. International Journal of Pharmaceutics, 455(1-2):159-168.
[18] A. U. Vanarase, M. Jarvinen, J. Paaso, and F. J. Muzzio. 2013. Development of a methodology to estimate error in the on-line measurements of blend uniformity in a continuous powder mixing process. Powder Technology, 241:263-271.
[19] V. Kumar, M. K. Taylor, A. Mehrotra, and W. C. Stagner. 2013. Real-time particle size analysis using focused beam reflectance measurement as a process analytical technology tool for a continuous granulation-drying-milling process. AAPS PharmSciTech, 14(2):523-530.
[20] Simeone Zomer, Manish Gupta, and Andy Scott. 2010. Application of multivariate tools in pharmaceutical product development to bridge risk assessment to continuous verification in a quality by design environment. Journal of Pharmaceutical Innovation, 5(3):109-118.
[21] N. Souihi, M. Dumarey, H. Wikstrom, P. Tajarobi, M. Fransson, O. Svensson, M. Josefson, and J. Trygg. 2013. A quality by design approach to investigate the effect of mannitol and dicalcium phosphate qualities on roll compaction. International Journal of Pharmaceutics, 447(1-2):47-61.
[22] R. D. Braatz, R. C. Alkire, E. G. Seebauer, E. Rusli, R. Gunawan, T. O. Drews, and Y. He. 2006. Perspectives on the design and control of multiscale systems. Journal of Process Control, 16:193-204.
[23] K.-K. K. Kim, D. E. Shen, Z. K. Nagy, and R. D. Braatz. 2013. Wiener’s polynomial chaos for the analysis and control of nonlinear dynamical systems with probabilistic uncertainties. IEEE Control Systems, 33(5):58-67.

